5300 - fold over - subscription, surpassing "Snow King", the concept stocks of weight - loss drugs soared 200% on the listing.
Text by | Hu Xiangyun
Edited by | Hai Ruojing
The upward trend of new innovative drug stocks continues.
On August 15th, Yinnuo Pharmaceutical, a GLP - 1 (Glucagon - like Peptide - 1) concept enterprise, was listed on the Hong Kong Stock Exchange. Its intraday increase reached 300% at one point and finally closed up 200%, with a market value exceeding 26 billion. One day before the listing, the gray - market trading of the company rose by more than 270%, and the new - share subscription was extremely popular. The public offering part was over - subscribed by 5364 times, even exceeding the 5257 - times over - subscription of Miss Fresh.
In the past two years since Semaglutide and Tirzepatide became popular, many enterprises have tried to list on the Hong Kong stock market with the GLP - 1 label. For example, Jiuyuan Gene and PaiGe Biotech were successfully listed before their products were approved, relying only on biosimilars or improved versions of foreign original drugs.
In contrast, Yinnuo Pharmaceutical has relatively outstanding "hard power", and some of its products have entered the commercialization stage. At the beginning of this year, Yisupaglutide α, a self - developed GLP - 1 drug for the treatment of type 2 diabetes in adults, was launched. By the end of May, its sales revenue was nearly 40 million yuan. In addition, the indications for weight loss and metabolic dysfunction - associated steatohepatitis, which have more potential, have entered the clinical stage.
The business aspect is the foundation, but it is also important to note that the biomedical sector is recovering, and the market is chasing the concepts of innovative drugs and "domestic GLP - 1". These factors together support Yinnuo Pharmaceutical's current business prospects and stock market performance.
Betting on Long - acting GLP - 1
In the development of injectable GLP - 1 drugs, less frequent dosing, better treatment effects, and stronger safety have become the recognized competitive directions. Especially with successful predecessors, it is difficult for later - comers to enter the GLP - 1 market if they do not have outstanding advantages in one of these indicators.
Specifically for Yinnuo Pharmaceutical, its "strength" lies in long - acting drugs.
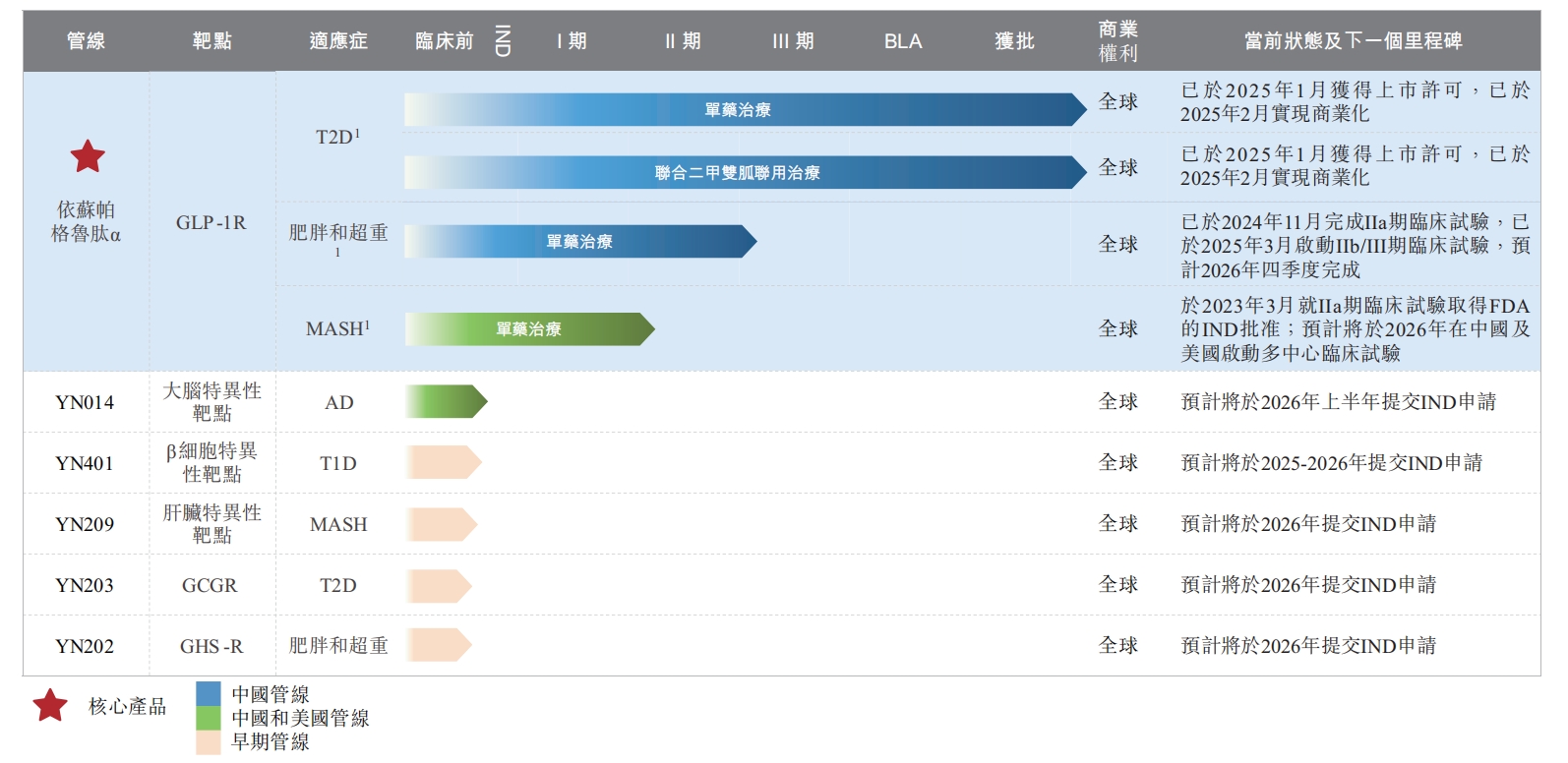
Yinnuo Pharmaceutical's product pipeline (Source: Prospectus)
Generally, GLP - 1 products with a continuous effect of more than 24 hours after a single use are considered long - acting preparations. Especially in recent years, with the successive launches of Semaglutide and Tirzepatide, the two major weekly preparations, the market scale of short - acting GLP - 1 drugs that require daily dosing has been rapidly squeezed. According to Sullivan data, in 2024, the market shares of long - acting GLP - 1 drugs globally and in China reached 96.5% and 86.9% respectively.
The research data disclosed in the prospectus shows that the half - life of Yisupaglutide α reaches 204 hours. In a horizontal comparison with the R & D data of currently announced GLP - 1 drugs, this figure exceeds the 168 hours of Semaglutide, the 120 hours of Tirzepatide, and the 112 hours of Dulaglutide.
In the previously launched indication for diabetes treatment, Yinnuo Pharmaceutical still stated that the dosing frequency of Yisupaglutide α is once a week, the same as Semaglutide and Tirzepatide. However, the "204 - hour" half - life means that Yisupaglutide α is expected to further reduce the dosing frequency.
Especially in the weight - loss indication, which has more consumer attributes, this may attract users to some extent.
However, some practitioners in the GLP - 1 field believe that in the weight - loss indication, such "optimization" may be a bonus, but the core evaluation criterion is still the weight - loss efficacy. At the end of last year, Yisupaglutide α completed the Phase IIa clinical trial for the weight - loss indication. The data disclosed by Yinnuo Pharmaceutical is not very detailed. It mentioned that after 4 weeks of treatment with 20mg of Yisupaglutide α, the average weight loss compared to the baseline was 8.13%, while that of the placebo group was 0.79%.
In March this year, Yisupaglutide α started the Phase IIb/III clinical trial, which is expected to be completed by the end of next year. More detailed effects of Yisupaglutide α may be revealed during this period.
Previously, the Phase III clinical trial of Eli Lilly's Tirzepatide showed that after 72 weeks of use, the weight - loss effects of the 15mg and 10mg dose groups were 20.9% and 19.5% respectively. Recently, Novo Nordisk also presented new research on the weight - loss effect of Semaglutide at the American Diabetes Association Scientific Sessions (ADA). At the doses of 2.4mg and 7.2mg, the weight loss of the subjects at the 72nd week reached 17.5% and 20.7% respectively.
In addition, the aforementioned practitioner mentioned that one notable aspect of Yisupaglutide α is "preserving muscle mass while losing weight". The muscle mass of the human body directly affects the basal metabolic rate. If muscle loss occurs during weight - loss treatment, it may lead to a decrease in the basal metabolic rate and an increased probability of weight rebound after stopping the drug. Therefore, companies focusing on the R & D of weight - loss drugs have gradually begun to emphasize the "muscle - building" benefits of their products during the clinical process.
At this year's ADA, Yinnuo Pharmaceutical announced the early cohort data of Yisupaglutide α, showing that "after 4 weeks of treatment, the average weight of the subjects decreased by 8.6kg, with a weight - loss rate of 8.6% and a fat - loss rate of 14.79%". Yinnuo Pharmaceutical explained that this result indicates that "fat was preferentially 'burned' during the treatment, and muscle tissue was almost completely preserved".
The Intense Competition in Weight - loss Drugs Has Arrived,
Can It Rank Among the Top?
Among the leading players in the weight - loss drug field, the core participants are multinational pharmaceutical companies. Most domestic enterprises, such as Innovent Biologics, Hengrui Medicine, and CSPC Pharmaceutical Group, are also pharmaceutical companies with long - term and mature commercialization experience. In contrast, how to stand out in the highly competitive metabolic drug market tests Yinnuo Pharmaceutical's commercialization ability.
According to the prospectus, Yinnuo Pharmaceutical has established a sales team of 15 people for Yisupaglutide α, with an average work experience of 20 years in the field of metabolic diseases. In the first five months of 2025, the company's sales expenses increased to 37.538 million yuan.
However, it is still difficult for Yinnuo Pharmaceutical to compete with domestic and foreign pharmaceutical giants, especially when the rules for drugs to enter hospitals are becoming stricter and many medical institutions have restrictions on the number of products with the same generic name. Negotiating with hospitals one by one is obviously not Yinnuo Pharmaceutical's strength.
Perhaps considering this, Yinnuo Pharmaceutical has attached great importance to off - hospital channels from the beginning in the indication for diabetes treatment, which is more suitable for in - hospital use. The company's prospectus particularly mentioned that "it attaches great importance to online e - commerce and Internet hospital channels". After the commercialization of the diabetes indication in February this year, "on a leading e - commerce platform, the search index increased by 40% in the second month".
In addition, a document provided by Yinnuo Pharmaceutical to 36Kr stated that during this year's "618" shopping festival, Yisupaglutide α ranked 7th in endocrine drugs and 3rd in GLP - 1 drugs on JD.com and Alibaba respectively.
As of May this year, the total revenue of Yisupaglutide α was 38.144 million yuan. These early efforts in channel development and testing will also lay the foundation for the future launch of the weight - loss indication.
It should be noted that extending the sales of GLP - 1 weight - loss products from in - hospital to off - hospital is not a unique strategy of Yinnuo Pharmaceutical but a consensus among all participants.
As soon as the weight - loss indications of Semaglutide and Tirzepatide were approved, they began to be available for pre - order on the three major e - commerce platforms: JD.com, Meituan, and Alibaba. Domestically, the GLP - 1 weight - loss product Liraglutide Injection of Huadong Medicine has been stocked in nearly 10,000 cold - chain stores, and Benaglutide Injection of Renhui Bio attaches great importance to DTP pharmacies and brand - chain pharmacies.
These are just the products that have already been approved for listing. According to statistics, including Yisupaglutide α, there are currently more than 50 GLP - 1 drugs in development for the weight - loss indication in China, and many of them are in the Phase II or III clinical stage. When they are approved in a concentrated manner, the market competitiveness of each product may be weakened.
Especially recently, although the global GLP - 1 market still looks prosperous in terms of sales data, for example, Semaglutide and Tirzepatide are still selling well, with sales of 16.6 billion US dollars and 14.734 billion US dollars in the first half of the year respectively. However, the industry has begun to take a more critical look at the prospects of the GLP - 1 weight - loss market.
After Novo Nordisk lowered its full - year performance guidance and Eli Lilly's new drug R & D data fell short of expectations, the stock prices of the two companies dropped back to the levels of the previous two years. In addition, recently, Pfizer announced the full termination of its GLP - 1 pipeline R & D, mainly based on a "comprehensive assessment of the GLP - 1 market competition pattern".
Moreover, some American researchers published a study at the beginning of this year, stating that more than 70% of GLP - 1 drug users stopped using the drugs in the past two years. Although the reasons for this phenomenon are complex, the industry generally believes that the access to the products and the convenience of dosing are important factors that directly affect whether users can continue using the drugs. Whether the long - acting potential of Yisupaglutide α can create a differentiated competitive advantage and achieve commercial benefits comparable to the "miracle GLP - 1 drugs" remains to be seen.