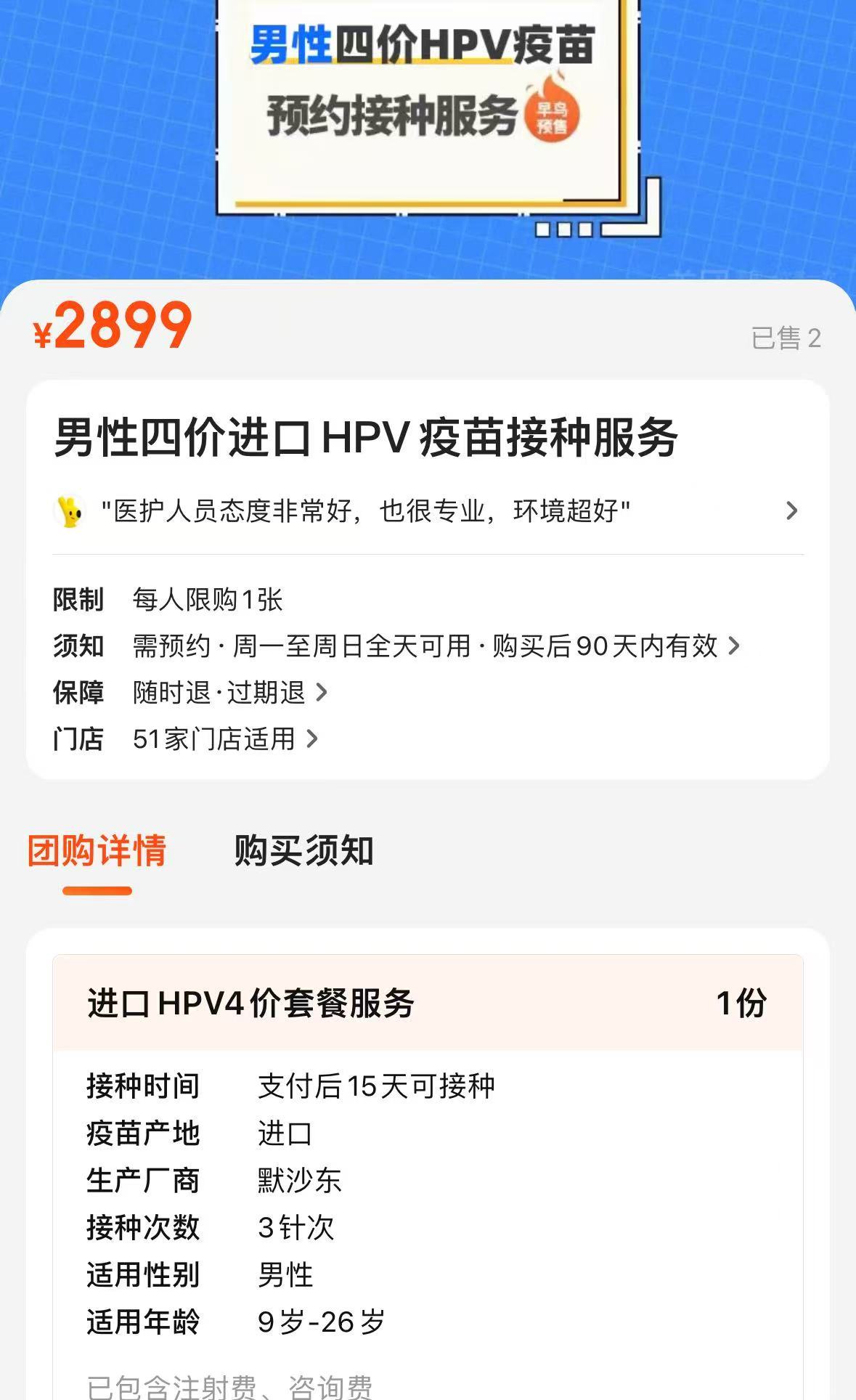
China's first HPV vaccine for men has been approved. Some e-commerce platforms are selling it at a price of 3000 yuan, but how many people are willing to get vaccinated? | Focus Analysis
In China, the male population can finally obtain the HPV (Human Papillomavirus) vaccine.
On January 8, Merck's quadrivalent HPV vaccine "Gardasil" was officially approved by the National Medical Products Administration, with the indication of vaccination for men aged 9 - 26 added, becoming the first and only approved male HPV vaccine in China. Among the fierce competitions of many domestic and foreign manufacturers, it is still the established foreign enterprises that ultimately come out on top.
Looking overseas, the male HPV vaccine is not a new market. After all, more than 70 countries have included it in the immunization program vaccination list (the government pays and mandatory vaccination). But in China, it is still a truly new story in the HPV vaccine track.
In 2018, Merck's nine-valent HPV vaccine exclusively represented by Zhifei Biological entered the domestic market. Under the hunger marketing, the self-paid product with a cost of several thousand yuan was once in short supply to the extent that "420,000 people entered the lottery for vaccination, and the winning rate was less than 1.7%". However, with the successive listing of several domestic bivalent products, the HPV vaccine market quickly entered the era of meager profits. By 2024, in some provinces, the government procurement price of a single vaccine has dropped to less than 30 yuan.
For vaccine manufacturers, since the female market has become a "cabbage price", it is a very reasonable logic to expand the male user group. According to Sullivan data, in 2020, there were 344 million young men aged 9 - 45 in China who were eligible for vaccination. Calculated by the full dose of three injections per person, there are nearly 1 billion injections.
This cake is hard not to be tempting. Currently, although Merck has not yet disclosed the price of "Gardasil", but on the first day of approval, some e-commerce platforms have been able to make an appointment for this product, and the price of three injections is 2,885 yuan. A customer service representative said, "In stock."
In the expected prosperity, sellers seem to have only avoided the question of "How to sell the male HPV vaccine": Are domestic men really willing to be vaccinated?
The appointment interface for male HPV vaccine on the e-commerce platform
To promote the male HPV vaccine, should we "convince" his partner first?
Convincing men to receive the HPV vaccine is not an easy task worldwide.
This point, the overseas market has actually given an answer. In 2023, an overseas data cited in a study in the Chinese General Practitioner Journal mentioned that since some overseas countries began to promote the HPV vaccine for both men and women simultaneously in 2014, until 2019, the vaccination rate and completion rate of men "were still very low", only 5% and 4% respectively. In contrast, these two figures reached 20% and 15% respectively in the female population.
In addition, overall, the cost-effectiveness of male HPV vaccination is also controversial. The study mentioned that if the vaccine price and coverage rate are low, including adolescent boys in the vaccination plan is cost-effective. But if the female coverage rate has exceeded 75%, the existing evidence is "insufficient".
This is certainly related to the "market education" of the manufacturers in the past to popularize HPV vaccination. In the past, the HPV vaccine has been regarded as a "female product" by the public. Women who pay attention to their own health and have a sense of self-protection should take the initiative to be vaccinated. But another reason that cannot be ignored is that although men are the main spreaders of the HPV virus factor, compared with women who may suffer from cervical cancer, the probability of men directly getting ill is much smaller.
Chen Jianguo, a business partner of the Healthcare Division of Hejun Consulting, has long been concerned about the vaccine industry. He mentioned that in terms of the awareness of vaccinating to prevent HPV infection and its potential risks, currently "men are generally weaker than women". On the one hand, the vaccination situation abroad is also dominated by the female market, and the male market has improved, but the proportion is limited. On the other hand, although men may cause cancers such as penile cancer or diseases such as condyloma acuminatum after infection, "but the overall situation is relatively mild", and the treatment methods after early infection are relatively mature, and the necessity of spending a lot of money to prevent by vaccination is relatively not high.
In other words, it may be slightly more difficult to directly use the original marketing strategy of "getting vaccinated to protect your health" to move the male group. A more reasonable logic may be to use the existing "stock market" of female vaccinators to "pry" the male user group.
Two years ago, Abby "pushed" her boyfriend to receive the HPV vaccine. At that time, the relevant product had not yet been approved in China, and the two made an appointment for vaccination in the school hospital when they were studying in the United States. But according to her observation, the awareness of the HPV vaccine among the male friends around her at most stayed at the level of "both men and women can be vaccinated", and few people would deliberately understand which male diseases the vaccine can prevent.
"In addition to being told by their partners that getting vaccinated is beneficial to health, the most important reason that affects their decision is probably that the student insurance that the school forces to pay is $10,000. In order to 'get the best deal', everyone almost got all the vaccines that they could." Abby analyzed.
In the past two years, this voice of "for her health" has also been heard on domestic social media. Some male bloggers who went to Hong Kong, Macao or overseas to be vaccinated have posted strategies, sharing appointment channels or a list of reliable medical institutions. There is even a slogan from netizens:
"HPV vaccine, the best dowry for men."
Chen Jianguo believes that after the relevant products start to be officially promoted, there is a possibility of using female users to drive the male market, but the conversion efficiency depends on the enterprise's marketing ability. If the marketing and education are not enough, the market may not be very high. "In addition, this also depends on the pricing and supply of the product. The hunger marketing strategy for the female market in the past is not suitable for the current male market promotion."
At the same time, there is also another voice in the industry that in recent years, with the World Health Organization (WHO) and China successively releasing and implementing the action plan to accelerate the elimination of cervical cancer, and the HPV vaccine being included in China's immunization program, it may also be one of the effective ways to expand the male market.
For example, Kangleweishi, a domestic manufacturer listed on the Beijing Stock Exchange and an early layout of the male HPV vaccine, optimistically predicted in its semi-annual report in 2024 that in the future, the HPV vaccine "may also be included in the national immunization program" in China, thereby improving the public's vaccination awareness and vaccine coverage.
In this regard, many respondents in the vaccine industry have given a relatively consistent view that it is difficult to achieve this goal in the short term. A more likely situation is that the high and low-price HPV vaccine markets present two differentiated market patterns. Low-price vaccines are mainly used for children's vaccination purchased by the government, while high-price vaccines are mainly for adult self-paid vaccination.
Previously, the research report of Kaiyuan Securities also mentioned that considering factors such as regional epidemiological characteristics, vaccine accessibility, economic development level and financial payment capacity, there may be a "long time gap" between the "recommended varieties by WHO and the varieties that China officially includes in the national immunization program".
Domestic manufacturers are gathering strength, is it another cycle of price war?
HPV vaccine manufacturers indeed have many helplessness when they pin their hopes on the male market.
It has only been a short seven years since 2018. In the medical industry, this should have been the prime time for a "blockbuster" drug. But the domestic female HPV vaccine has shown a trend of being difficult to sell in 2024.
Let's first look at Merck. The Chinese market can basically contribute 60% - 70% of the international sales of HPV vaccines for it. In the first three quarters of this year, the overall sales of HPV vaccines were 2.306 billion US dollars, a year-on-year decrease of about 10%. The main reason is the reduced demand in the Chinese market. In addition, other regions around the world basically maintain double-digit growth. Currently, although Merck has reduced the shipment of HPV vaccines to Zhifei Biological, the overall inventory is still very high.
In addition, under the "price war" and the government's free vaccination program, the female bivalent HPV vaccines that domestic vaccine manufacturers have concentrated on listing are also difficult to be called a good business. Taking the representative enterprise Watson Biotech as an example, in 2022, after its bivalent HPV vaccine was approved, the company's annual revenue also reached the peak in recent years, approaching 5.1 billion yuan. But in the first three quarters of this year, the company only achieved an income of 2.141 billion yuan, a year-on-year decrease of 32.19%, mainly due to the "reduction in vaccine product sales revenue".
The same is true for another company, Wantai Biological. Although the company has several vaccines under research, its income is mainly driven by the bivalent HPV vaccine. In the first three quarters of this year, Wantai Biological's income was 582 million yuan, a year-on-year decrease of 60.79%. The company stated that the vaccine sector was affected by market adjustments, government centralized procurement and the expanded age range for the nine-valent vaccine, and the sales revenue decreased compared with the same period last year.
Chen Jianguo analyzed that in the face of the current fiercely competitive situation in the female HPV vaccine market, it is time for HPV enterprise practitioners to adjust the product medical strategy and market strategy.
Now, each enterprise actively turns to the male vaccine direction in response to the market, which can be regarded as a new opportunity. After "Gardasil", there are many male HPV vaccines queuing for clinical trials and waiting for listing, and they are mainly nine-valent products.
Those with rapid progress, in addition to Merck's nine-valent HPV vaccine that has entered the acceptance stage of the listing application, the nine-valent vaccines of Wantai Biological, Kangleweishi and Bowei Biological have all entered the third phase of clinical trials. Among them, Kangleweishi once stated in the prospectus that the male nine-valent HPV vaccine is expected to be listed in 2028.
Overall, The R & D progress of the male HPV vaccine products of leading enterprises is relatively unified, and a concentrated listing period may be ushered in in the future.
It is worth noting that the approval of HPV vaccines basically goes through a process of expanding from a small range of age groups to a larger group. Just like Merck's female nine-valent vaccine, it was not until four years after its listing that the applicable age range changed from 16 - 26 years old to 9 - 45 years old. And the vaccination awareness of the male group for HPV vaccine is not strong. In addition, the currently approved age group of 9 - 26 years old mostly does not have the ability to pay independently. The real "volume increase" may have to wait until the indication for the age group after 26 years old is approved.
It is just unknown whether the "price war" story in the female HPV vaccine market will be staged again in the male vaccine market at that time?