"The Worst" Affluent Disease: After a Ten-Year Wait, a New Drug Finally Arrives | Zhiliao
When it comes to metabolic diseases, many people probably first think of diabetes and hyperlipidemia. Over the past few years, both pharmaceutical companies and investment institutions have invested countless money and energy in this area. From semaglutide (GLP-1), a blockbuster drug that single-handedly drives the entire GDP of Denmark, to inclisiran sodium injection, a long-acting lipid-lowering drug that can still attract 200 new patients every day even with a price of ten thousand yuan, metabolic diseases have supported one after another tens of billions of markets in the pharmaceutical industry.
However, compared to these diseases that attract a lot of attention, few people have noticed that for gout and hyperuricemia, which have already become the second-largest metabolic disease, there have been no new drugs on the market for nearly 10 years.
According to a research report from Ping An Securities, with the change in diet structure and lifestyle, the prevalence of gout in China is continuously increasing. In 2020, the number of patients with gout and hyperuricemia in China was about 180 million, and this number may reach 240 million by 2030, and "the onset age is becoming younger and younger".
If viewed purely from the treatment perspective, gout is not incurable. It's just that the products in the past had more or less some side effects. Until recently, a drug called Dotinurad Tablets under Eisai was approved for listing in China for the treatment of gout with hyperuricemia, and this "vacancy period" has finally come to an end for the time being.
How will the arrival of the new drug stir the gout market?
Gout Drugs: A New Addition After Ten Years
The occurrence of gout is highly related to dietary habits. This is why in the past, gout was often considered a "disease of the rich" that only people with good living conditions would get. But now, with the improvement of living conditions, office workers are fond of barbecue and beer, and young people love to drink "sugary drinks", and gout has gradually begun to "attack" people of all ages equally.
If we delve into the cause of its occurrence, the deposition of uric acid is the cause, and the occurrence of gout is the result. In the human body, the deposition of urate crystals will stimulate the body to activate the defense mechanism, and white blood cells will engulf the urate crystals, leading to the release of inflammatory chemicals, thereby causing related symptom reactions.
Therefore, inhibiting the production of uric acid has become one of the most effective ways to treat gout, and these drugs are collectively called xanthine oxidase inhibitors (XOI). Currently, the most commonly used treatment drug on the domestic market, febuxostat, follows this principle. After its approval in 2013, the market performance of this drug has been consistently strong. Until 2020, when it encountered centralized drug procurement, the price dropped from about 10 yuan per tablet (40mg specification) to about 1 yuan, directly leading to a "shrinkage" of the market.
According to statistics from the PDB database, in sample hospitals mainly composed of secondary and tertiary hospitals, the sales of febuxostat were still 400 million yuan in 2020. But by 2021, it had directly dropped to just over 100 million yuan.
But after all, the ultimate direction of the price reduction is beneficial to patients. The real problem that makes the outside world hesitate is that XOI drugs represented by febuxostat have always had the problem of poor efficacy. Relevant statistics show that about 40% to 60% of patients cannot effectively control the uric acid level after taking the medicine; in addition, the safety of this drug is also controversial. In 2019, the US FDA even directly issued a black box warning for it, requiring that the drug instructions be marked with "an increased risk of cardiovascular death, and there are risks of gout attacks, hepatotoxicity, and severe skin allergies" and other issues.
In other words, taking it may harm the liver, and not taking it may harm the kidneys.
But now, with the listing of Eisai's Dotinurad, gout "patients" may no longer need to be in this predicament.
In China, Eisai has conducted a phase 3 clinical study aimed at evaluating the efficacy of Dotinurad and febuxostat in the treatment of gout. This study included a total of 451 gout patients and randomly divided them into the Dotinurad group (4mg dose) and the febuxostat group (40mg dose), comparing the percentage of patients with a blood uric acid level of ≤6.0mg/dL after 24 weeks of treatment with the two drugs.
The results showed that in this study, after treatment with 4mg Dotinurad, the proportion of patients with a blood uric acid level of ≤6mg/d (360μmol/L) at 24 weeks was 73.6%, while this figure in the febuxostat group was only 38.1%; in addition, another phase 3 clinical study previously conducted in Japan also showed that after 58 weeks of treatment with Dotinurad in patients with hyperuricemia (with or without gout), the proportion of patients with a blood uric acid level of ≤6mg was 100%.
In other words, Dotinurad has a higher uric acid compliance rate than febuxostat. More importantly, long-term use of Dotinurad has no obvious effect on renal function and no clinically relevant effect on liver function.
For a long time, gout patients have actually been in a state where their clinical needs have not been met. This "blank" is bound to create a new metabolic blue ocean market, and quickly breaking through the safety problem will inevitably become the key to winning in it.
Judging from the current market performance, Dotinurad may be the one with the greatest potential.
Behind Dotinurad: 50 Years of a Star Gout Target
Why Dotinurad can achieve a "leap" in therapeutic effect probably needs to start from the mechanism behind it.
Both act on uric acid, but the mechanisms are different. If XOI drugs such as febuxostat prevent the production of uric acid from the source; then, uricosuric drugs represented by Dotinurad are trying to make the already produced uric acid "disappear".
Specifically, Dotinurad mainly inhibits uric acid reabsorption and reduces blood uric acid levels by selectively inhibiting the urate transporter 1 (URAT1) related to uric acid reabsorption in the kidneys. Initially, this drug was developed by Fuji Pharma, and later, Eisai obtained the exclusive rights to develop and sell it in China in 2020.
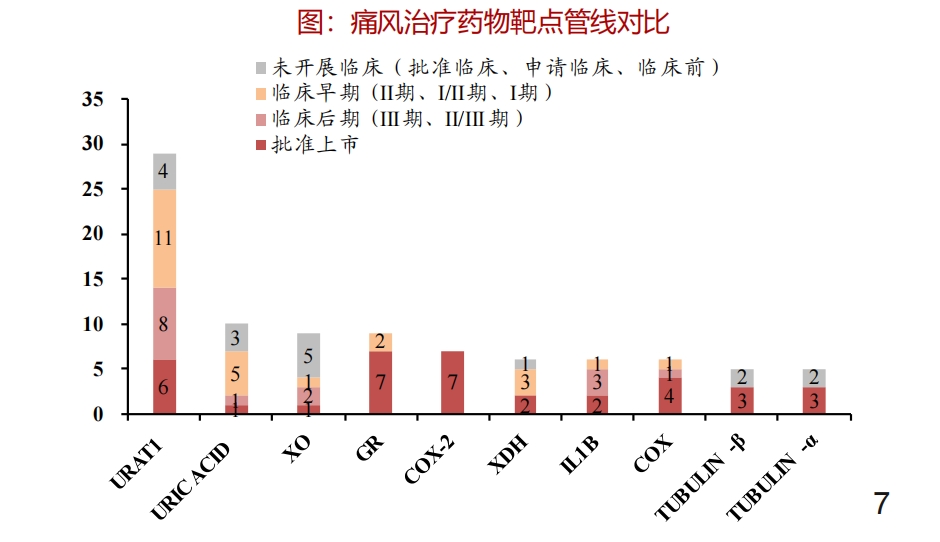
This is because in the human body, the role of URAT1 is to be responsible for "absorbing most of the urate", which can mediate about 90% of uric acid reabsorption, and compared with other transporters, it has a higher affinity and transport efficiency for uric acid, which also makes URAT1 one of the hottest targets in the history of hyperuricemia/gout drug research.
According to the research report data from Zheshang Securities, currently, there are about 30 URAT1-type gout treatment products in the stage of research and development or have been approved for listing, which is much higher than drugs with other types of targets.
Source: Zheshang Securities
Although today, the representative variety of uricosuric drugs is undoubtedly Dotinurad, but in the nearly 50 years since the URAT1 target first appeared on the historical stage, in fact, many products have repeatedly experienced listing and delisting, and the core reason is actually because of safety.
In 1971, the first URAT1 inhibitor, benzbromarone, was approved in Germany. But later, as the product's global marketing expanded, liver toxicity and other problems were reported in many countries such as the Netherlands and Japan. Therefore, the US FDA, as the global drug review benchmark, has always rejected this drug due to the problem of "serious risk of liver damage". Around 2000, benzbromarone was delisted in a lonely manner.
In fact, it was during this "silent" period that XOI drugs briefly became a research and development hotspot in the field of gout. Until 2015, the emergence of the second global URAT1 inhibitor, lesinurad, made the medical community once again see the hope of the URAT1 target.
Because the clinical data disclosed by lesinurad at that time showed that the drug not only had a significant effect, but also had good safety, and could even be directly used in patients with renal failure.
This eye-catching data directly attracted the attention of pharmaceutical giants. The original research manufacturer of lesinurad was later acquired by AstraZeneca for nearly 1.3 billion US dollars, and its me-too drugs have also become the target of followers' layout.
But in the end, lesinurad also failed to escape the safety defect and was greeted with the fate of a black box warning from the FDA.
Interestingly, during this period, the cause of benzbromarone's hepatotoxicity was gradually confirmed and modified by the scientific community, and the drug designed with a new structure was reintroduced as "Dotinurad".
After going around in a big circle, it finally drew a successful conclusion to the story.
The development history of the URAT1 target is actually a microcosm of the research and development of many types of drugs in the pharmaceutical industry. Constant overthrow, reconstruction, advancement, and transcendence may be the true meaning of "follow".
Many Domestic Pharmaceutical Companies Enter the Market, Intense Competition in New URAT1 Inhibitors
The fateful entanglement of the three first-generation products has ended, but the exploration of the URAT1 target is actually continuing. And Chinese pharmaceutical companies have now become important participants in it.
A research report from Ping An Securities shows that currently, there are at least 20 small molecule drugs for the treatment of hyperuricemia/gout under research globally, with URAT1 inhibitors as the main ones. Among domestic enterprises, the enterprises with advanced research and development progress of URAT1 inhibitors mainly include Hengrui Medicine's SHR4640 (domestic Phase III), Yingli Pharma's YL-90148 (domestic Phase III), SinoVac Biotech's XNW3009 (domestic Phase III clinical), and New Element Pharma's ABP-671 (domestic Phase IIb/III clinical), etc.
Source: Ping An Securities Research Report
Behind these players, one can actually see the shadows of the "first generations" to some extent.
For example, Hengrui Medicine's SHR4640 is derived from the modified structure of lesinurad. In 2019, its clinical trial of using the single drug to treat patients with primary gout with hyperuricemia has already reached the Phase III clinical stage, but the updated progress has not been disclosed; in 2022, the drug also initiated a Phase II clinical study in combination with febuxostat. The industry speculates that this trial may be intended to "solve the safety problem through combination therapy".
Another example is SinoVac Biotech's XNW3009 and Dotinurad. The Phase II clinical data show that the 0.5mg dose group of XNW3009 can achieve a serum uric acid concentration of ≤360umol/L in more than 72% of the subjects with each administration. The effect is already better than benzbromarone as the control group, and the safety and tolerance are good. Most of the adverse events are mild adverse events of grade 1 to 2, and no obvious hepatotoxicity or nephrotoxicity is observed.
On the other hand, in addition to these "improved" products, more explorations for other mechanisms of action are also underway. For example, targeting the IL-1 family is regarded as a "new choice for the treatment of gouty arthritis". This is because a large number of inflammatory cytokines such as IL-1α and IL-1β are produced in the inflammatory response of gout.
At present, enterprises using this research route include GenSci Pharmaceuticals, 3SBio, etc., and they have basically reached the Phase III clinical stage.
It can be foreseen that the market for gout drug treatment is becoming more and more abundant. The next blockbuster product may be among them.
Reference Materials:
Ping An Securities "Panoramic Map of Hyperuricemia and Gout Industry - Tens of Millions of Gout Patients in China Are in Urgent Need of New Drugs with Good Efficacy and Safety"