7 billion! A 30-year-old "fourth-generation scion of a wealthy family" acquires a Shanghai innovative pharmaceutical company.
Text | Hu Xiangyun
Editor | Hai Ruojing
"As of last Friday's board meeting, the R & D of the two drugs in the BD pipeline of Lixin Pharmaceutical was progressing normally, and multinational pharmaceutical companies attached great importance to them." At the M & A briefing on July 17, Xie Qirun, the chairman of the board of China Biopharmaceutical (01177.HK, hereinafter referred to as "China Biopharmaceutical"), responded to the market rumor that Lixin Pharmaceutical's products might be returned.
Just three days ago, China Biopharmaceutical announced that it would acquire 100% of the shares of domestic innovative drug company Lixin Pharmaceutical for a consideration of $950 million (approximately RMB 6.8 billion). After the acquisition is completed, the Lixin Pharmaceutical team will join China Biopharmaceutical collectively, and "corresponding incentives will be given".
Lixin Pharmaceutical is a current star innovative drug company. Since its establishment in 2019, it has basically maintained a financing pace of once a year. Behind it, there are not only investors such as Tigermed, Qiming Venture Partners, Qingsong Capital, and Shanghai Biomedical Fund, but also BD transactions with a total value of over $4 billion. Just the upfront payments and recent milestones of the BD deals with AstraZeneca and Merck alone amount to as much as $1 billion.
Why could China Biopharmaceutical acquire such an innovative drug asset for less than $1 billion? The outside world began to have wild speculations. There were rumors that Merck might "return the goods", and the value of Lixin Pharmaceutical's pipeline was actually not high.
It was not until the briefing on the 17th that the top leaders of both the buyer and the seller stood up simultaneously to deny it, saying that this was a transaction with "strong willingness and mutual pursuit", and it only took two months from negotiation to completion. Xie Qirun also said that based on the due - diligence results, she was "very confident" that the second payment from Merck would be received "in the near future".
High - cost performance: Acquire the dark horse in the innovative drug field
At the briefing, Xie Qirun joked that she got to know Qin Ying, the founder of Lixin Pharmaceutical, last year. The two are friends and comrades - in - arms, and they can "chat freely about their dreams" every time they meet.
It was also at the end of last year that China Biopharmaceutical and Lixin Pharmaceutical disclosed their first cooperation: as the leading investor in Lixin Pharmaceutical's Series C1 financing, China Biopharmaceutical acquired a 4.91% stake in Lixin Pharmaceutical for RMB 142 million and reached cooperation on multiple pipelines such as its core anti - CCR8 monoclonal antibody.
At that time, the topic of M & A was mentioned many times in a joking way. This year, as the innovative drug market recovered and after the two parties had a certain cooperation foundation, they began to formally promote the M & A.
Judging from the results, this M & A is highly cost - effective. The buyer said, "All stakeholders are very satisfied."
From China Biopharmaceutical's perspective, although the consideration is $950 million, after excluding the $450 million in cash and cash equivalents on Lixin Pharmaceutical's books, the net transaction price is only $500 million. Moreover, in China Biopharmaceutical's calculation, this amount should be further reduced by the pre - closing operating expenses totaling over $300 million and the first milestone payment from Merck. It is equivalent to that China Biopharmaceutical only spends a maximum of $200 million to gain full control of Lixin Pharmaceutical.
For the investment institutions behind Lixin Pharmaceutical, exiting through M & A is also a good choice. Based on the Series C1 financing in October last year, Lixin Pharmaceutical's valuation at that time was about RMB 2.9 billion. Now, it is sold for RMB 6.8 billion in less than 10 months. The return rate of the latest round of investment institutions will exceed 100%. The early - stage old shareholders who entered at a lower valuation may have even higher returns.
So, did the seller, Lixin Pharmaceutical, suffer a loss? Not necessarily.
Previously, Lixin Pharmaceutical was a rather low - key innovative drug enterprise and rarely made public statements. Qin Ying once studied under Academician Wang Zhenyi of the Institute of Hematology, Ruijin Hospital, Shanghai. After entering the industrial circle, she has successively worked in enterprises such as GlaxoSmithKline and Saisheng Pharmaceutical, with nearly 30 years of work experience.
Currently, Lixin Pharmaceutical is mainly engaged in the R & D of macromolecular innovative drugs. Both the ADC and bispecific antibody technology platforms have been maturely established. The BD transactions with AstraZeneca and Merck are the best proof: in 2023, Lixin Pharmaceutical licensed the anti - GPRC5D ADC drug LM - 305 to AstraZeneca for a recent payment of $55 million and a total price of $545 million; in November 2024, the company sold the PD - 1/VEGF bispecific antibody LM - 299 to Merck for an upfront payment of $888 million, technology transfer milestones, and a total price of $2.4 billion.
As of now, Lixin Pharmaceutical is also the only Chinese innovative drug enterprise that has reached BD cooperation with multinational pharmaceutical companies on both ADC and PD - 1/VEGF bispecific antibody, two types of popular products.
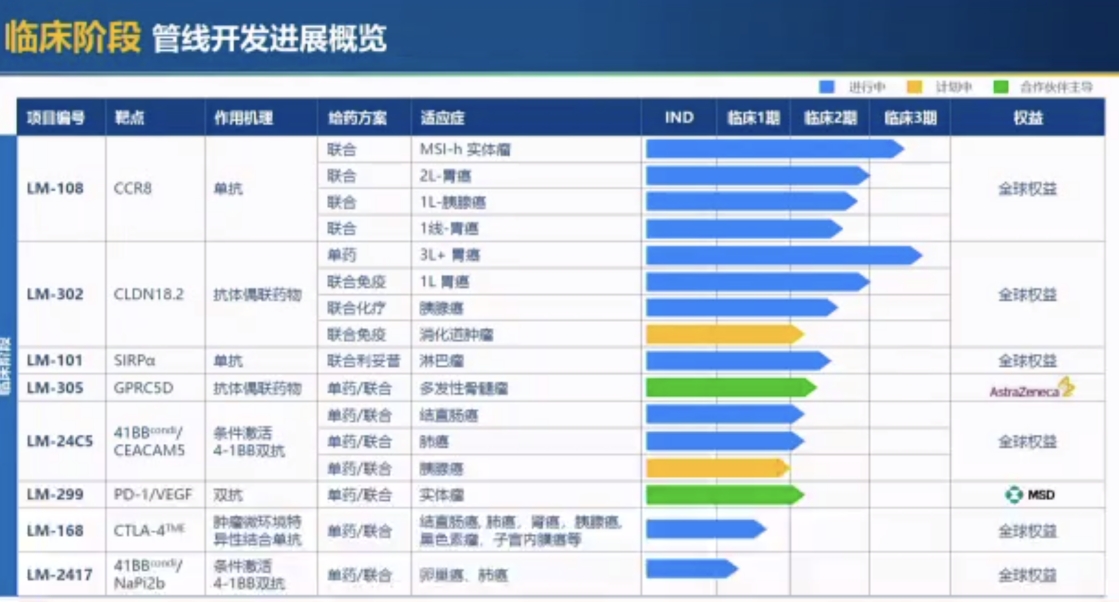
But on the other hand, like all domestic innovative drug enterprises, as the product pipeline progresses, clinical development ability is an unavoidable threshold. Since its establishment more than five years ago, the Lixin Pharmaceutical team has only 58 people so far. The clinical team accounts for about half of the total, but eight self - developed innovative drug pipelines have entered the clinical stage.
Among them, just the core CCR8 monoclonal antibody LM - 108 and the ADC drug LM - 302 targeting CLDN18.2 have simultaneously launched eight clinical trials, including two large - scale Phase III trials, and most of them are combination therapies. Moreover, both products are expected to be launched in the market in the next two to three years.
The development progress of Lixin Pharmaceutical's clinical - stage pipelines
To promote clinical trials of this scale requires huge amounts of funds and sufficient manpower. Innovative drug listed companies in the same stage in the industry often raise billions of RMB in financing. That is to say, with Lixin Pharmaceutical's current personnel scale and capital reserve, it will face extremely heavy pressure if it continues to operate independently.
Qin Ying also admitted that Lixin Pharmaceutical's current progress largely depends on CROs. In addition, from the very beginning, the company did not plan to conduct commercialization independently, so it has been looking for potential cooperation partners.
However, in the current market situation, although the M & A path is often mentioned, the era of large - scale M & A among Chinese innovative drug enterprises has not yet arrived. Looking for multinational pharmaceutical companies as buyers means huge integration costs and a long - term team - building process; looking at domestic enterprises, there are very few comprehensive enterprises that can "spend more than $200 million at one time on Biotech M & A" and have strong clinical and commercialization capabilities.
In other words, even a dark horse in the innovative drug field like Lixin Pharmaceutical has limited options at present.
"Joining China Biopharmaceutical is not a sudden decision. After in - depth investigation, it is the best choice," Qin Ying said.
Where will China Biopharmaceutical's innovation layout go?
The actual controller behind China Biopharmaceutical is the Xie family, a well - known overseas Chinese family in Thailand. The most well - known move of the company to the outside world is probably its investment in Sinovac Biotech in late 2020. Now, under the leadership of Xie Qirun and Xie Chengrun, the fourth - generation members of the "Xie family", this established traditional pharmaceutical enterprise is gradually promoting its "innovation transformation" process.
In June this year, China Biopharmaceutical announced that the revenue share of innovative products had increased from 16% in 2018 to 42% in 2024. By 2025 and 2027, this figure will increase to 50% and 60% respectively. In the next three years, following the path of "self - R & D+BD", it will launch five innovative products each year. In addition, China Biopharmaceutical's R & D investment in 2024 reached RMB 5.49 billion, of which the investment in innovative drug R & D accounted for nearly 80%.
Specifically, on the one hand, China Biopharmaceutical is gradually divesting its traditional business. For example, through selling partial equity in subsidiaries of the commercial circulation and generic drug sectors, it has accumulated more than RMB 2 billion in proceeds; on the other hand, it is accelerating the R & D of innovative products through BD and M & A.
Judging from China Biopharmaceutical's current product layout, according to the 2024 annual report, the company has 36 candidate drugs in the oncology field in the clinical or above - clinical development stage, among which three have entered the marketing application stage, and eight are in the Phase III/registration clinical stage. In terms of technology layout, compared with small - molecule drugs, the company has more obvious advantages in macromolecular drugs.
Schematic diagram of China Biopharmaceutical's technology platform
After the integration of Lixin Pharmaceutical's technology platform and products, it will undoubtedly further strengthen China Biopharmaceutical's R & D capabilities in this field. According to the current progress, the most anticipated is the CCR8 monoclonal antibody LM - 108, which was already under cooperation before the acquisition.
This is a chemokine receptor expressed on the surface of tumor - infiltrating regulatory T cells. Currently, although there are dozens of enterprises globally deploying this target, such as BMS, AbbVie, BeiGene and other domestic and foreign enterprises, no product has been approved, and only a few have disclosed data. Globally, LM - 108 is at the forefront of development, having obtained two breakthrough therapy designations, and is expected to be launched in 2028.
When introducing its clinical data, Qin Ying mentioned that in the treatment of solid tumors, gastric cancer, and pancreatic cancer, LM - 108 has shown the potential to be superior to standard treatments. Taking advanced solid tumors as an example, the median progression - free survival period after treatment with LM - 108 in combination with PD - 1 was 8.1 months, while that of standard treatment was only 3 months.
In addition to the products that have entered the clinical stage, the two parties will also accelerate the R & D of products in the earlier stages. China Biopharmaceutical said that Lixin Pharmaceutical's pipeline will become an "important early - stage R & D asset for the company in the future", and it is expected that multiple projects will enter the clinical stage in the next one to two years.
"This year, China Biopharmaceutical's market value has doubled. I hope that with the support of Lixin Pharmaceutical, it can quickly return to its peak and take off immediately," Xie Qirun said.