Exclusive AI supplier of a digestive endoscope manufacturer with a market share of over 70%. The leading investor in the new round of tens of millions of financing has passed the investment decision | First reported by 36Kr
Author | Zhao Siqi
Editor | Yuan Silai
Yingke learned that the leading investor in the new round of tens of millions of financing for Xiamen Inno Medical Technology Co., Ltd. (hereinafter referred to as "Inno Medical") has passed the investment decision. The funds from this round will be used for product certification and registration, R & D of new product lines, and overseas market expansion.
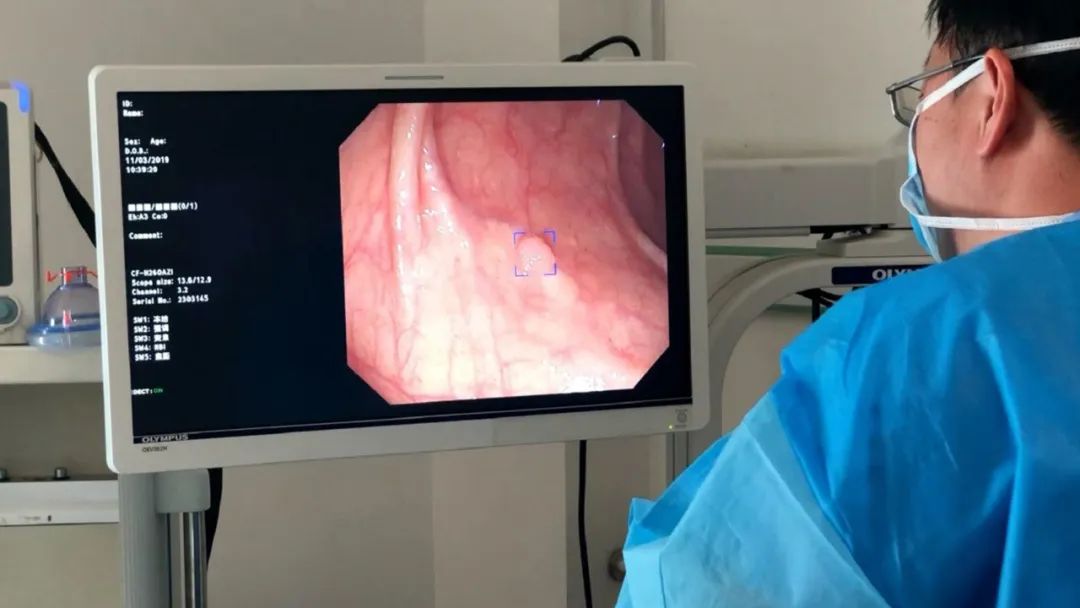
Since its establishment in 2017, "Inno Medical" has been focusing on the R & D and application of artificial intelligence solutions in the field of digestive endoscopy. Its core product, Inno Eagle Eye, was the first in the industry to build an AI-assisted system for the entire digestive tract endoscopy. It focuses on digestive endoscopy-assisted diagnosis, visual enhancement systems, and endoscopy operation quality control systems. It can identify lesions in real - time during hospital digestive endoscopy examinations and provide auxiliary judgments on the lesion type, differentiation degree, and infiltration depth. In 2023, the company reached a cooperation with Olympus (China), which has a market share of over 70% in the domestic digestive endoscopy field, and became its exclusive supplier of AI solutions.
Affected by factors such as strong subjective dependence on technology and operation, difficulty in identifying early digestive tract lesions, and a large patient base, digestive endoscopy examinations in China face many challenges. In traditional digestive endoscopy examinations, the average detection rate of intestinal adenomas is less than 20%, lower than the 25% baseline level in the United States; the average detection rates of early gastric cancer and esophageal cancer are only 20% and 30% respectively, less than one - third of those in Japan and South Korea. "Inno Medical" established an early cancer screening model based on the big data of digestive endoscopy centers. Combining the diagnosis and treatment thinking of digestive endoscopy experts, it conducts dynamic analysis of images to assist in the early identification and diagnosis of gastrointestinal tumors and precancerous lesions. In addition, the company's innovative Insight visual enhancement system uses generative AI technology to simulate the staining effect of partial pigment endoscopy, helping doctors analyze lesions, obtain auxiliary diagnostic information, and confirm the lesion boundary. While improving the diagnostic efficiency, it also reduces the doctor's operation time and consumable costs.
(Image source/Enterprise)
In terms of product layout, since the upper digestive tract involves multiple sub - regions such as the esophagus, stomach, and duodenum, it increases the difficulty of identification. At the same time, compared with the relatively regular lesion morphology of the lower digestive tract, the early lesions of the upper digestive tract have complex morphologies and lack typical features, making the R & D of the corresponding AI system very difficult and becoming a "bottleneck" problem in the industry. The gastroscopy - assisted system developed by "Inno Medical" is about to enter the clinical registration stage. It supports the detection and real - time boundary delineation of early esophageal cancer, gastric cancer, and their precancerous lesions; multi - disease endoscopic grading diagnosis; and prediction of the risk of gastric cancer. After nearly 20 domestic and overseas hospitals conducted clinical verification on the system, the feedback results showed that the detection accuracy of early lesions reached 2.6mm, and the detection rate of early esophageal cancer increased by about 20% with its auxiliary diagnosis.
Based on product compliance construction, "Inno Medical" obtained the first domestic Class III medical device registration certificate for the detection of lower digestive tract polyps under multiple light sources in 2023, which promoted the large - scale commercialization of this product ahead of the market. Clinical application feedback shows that the sensitivity of this intestinal polyp detection system to complex high - risk lesions such as broad - based and flat polyps is leading among foreign similar products, and it also reduces the interference of false positives to doctors. With its auxiliary detection, the average detection rate of intestinal polyps increased by about 10%. Research shows that about 95% of colorectal cancers develop from intestinal polyps, so an increase in the detection rate of intestinal polyps also means effective prevention and control of colorectal cancer.
In specific operations, the AI - assisted system is equivalent to a "real - time assistant" for endoscopists, prompting the examination coverage rate, withdrawal time and speed, and examination blind spots. It helps doctors in primary hospitals conduct standardized endoscopy examinations and diagnoses through quality control and navigation. Currently, the Inno Eagle Eye system has participated in the early screening public welfare projects for high - risk groups of colorectal tumors in county - level hospitals in Fujian, Jiangsu and other places.
While continuously expanding the clinical data set, the Inno Eagle Eye system combines the latest medical research results and collaborates with front - line doctors for detailed data annotation to improve the generalization and accuracy of the algorithm. In addition, the prior and simultaneous recognition rate of AI in auxiliary diagnosis, as one of the manifestations of the clinical value of the model, reaches 95% in the application of the "Inno Medical" colonoscopy - assisted system.
Hu Yanxing, the founder, believes that "a good clinical - end AI - assisted system makes doctors unaware of the existence of AI." Through a plug - in design, the Inno Eagle Eye system penetrates into the information path of the digestive endoscopy center and realizes transparent display on the original UI interface of the digestive endoscopy machine, fitting the traditional diagnosis and treatment process of doctors.
In terms of commercialization, the company reached an exclusive cooperation agreement with the leading endoscope manufacturer Olympus (China) and adopted a distribution model to sell its products together with the other party's endoscope system. Currently, the Inno Eagle Eye system has been installed in nearly 170 domestic and overseas hospitals, assisting in more than 250,000 diagnoses. The company estimates that the sales volume of the main units is expected to exceed 300 in 2026.
(Image source/Enterprise)
In the future, in addition to the field of digestive endoscopy, "Inno Medical" will expand the R & D of new product lines such as endoscopic ultrasound and respiratory endoscopy. At the same time, the company has planned to obtain the US FDA registration certification and plans to cooperate with domestic endoscope manufacturers to expand overseas markets.
Investor's view:
Li Ning, Investment Manager of Guohong Jiasin Capital: Inno Medical is an AI project we invested in 2020. In the past two years, when looking at generative AI, I have a view: don't do things that the future capabilities of large models will cover in entrepreneurship. Inno Medical is such a project. Its team has accumulated a large amount of medical record data in major tertiary - grade A hospitals across the country, and the deep barrier formed by this is difficult to be covered by the capabilities of general models. After our investment, Inno obtained the first Class III certificate in the lower digestive tract field in the industry in 2023. At the same time, it reached a cooperation with Olympus, which has a market share of over 70% in the digestive endoscopy field, and has become a leading player in the field of AI - assisted digestive endoscopy diagnosis.