"Core Medical" Secures Over $100 Million in Series D Financing, Completes Over 700 Artificial Heart Implant Surgeries in Total | Exclusive Report by 36Kr
36Kr has learned that CoreMedTech, an enterprise in the artificial heart field, has completed a Series D financing round exceeding $100 million. This round of financing was jointly led by ZhenFund, the Zhongguancun Special Fund for Independent Innovation of the National Social Security Fund (managed by Legend Capital), Prosperity7 Ventures (P7) under Aramco Ventures, CStone Capital, and well - known industrial investors, with DL Capital and LX Capital participating as follow - on investors. The raised funds will be used for innovative product development and to promote the company's international strategic layout.
CoreMedTech was founded in 2016 and focuses on the R & D of high - end innovative medical device products such as artificial hearts and mechanical circulatory support devices. Its product pipeline covers two major fields: implantable (LVAD, Implantable Left Ventricular Assist Device; BiVAD, Implantable Biventricular Assist Device) and interventional (pVAD, Interventional Ventricular Assist Device). It is an enterprise that 36Kr has been tracking and reporting on for a long time. This time, the Series D financing completed by the company is also the largest financing in the domestic innovative medical device field since 2025.
The shortage of heart donors is the biggest difficulty in the treatment of heart failure patients. In China, the number of heart transplant surgeries completed each year is less than a thousand, and there are fewer than 80 medical institutions qualified for heart transplantation. However, when considering the demand side of about 16 million patients with chronic heart failure, and calculating according to the 6% progression rate of refractory end - stage heart failure, the number of patients waiting for transplantation can reach nearly one million.
This thousand - fold difference constitutes a hundred - billion - yuan imagination space for the current heart failure device market in China. This is also the reason why artificial heart concept enterprises have been favored by capital in recent years.
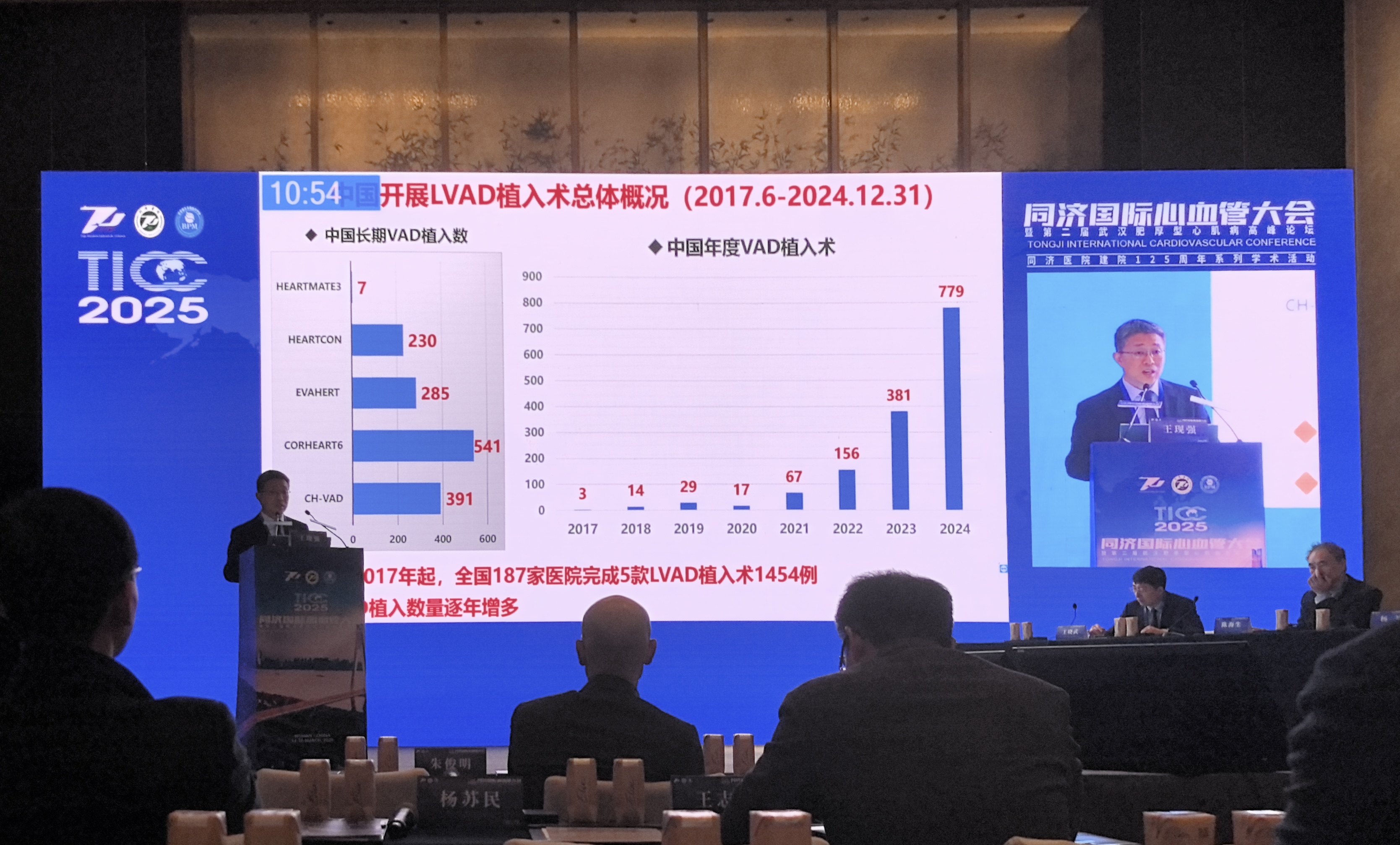
Tongji International Cardiovascular Congress (TICC) in 2025
CoreMedTech told 36Kr that in the field of implantable products, although the company's products do not have an advantage in terms of approval time, the technical barrier is more crucial in the high - end implantable medical device field. Currently, the company's core implantable left ventricular assist device, Corheart 6, uses axial full - magnetic levitation design and control technology, and has the characteristics of being “smaller and lighter” than similar products. The weight of the product pump body is only 90g, and the weight of the peripheral device is 800g, but the battery life reaches 33 hours.
As of now, Corheart 6 has completed more than 700 implantation surgeries in total.
However, current artificial heart products mainly refer to left ventricular assist devices. Among end - stage heart failure patients, more than 30% of patients with end - stage right - heart and biventricular failure currently have no mature long - term products available. Previously, some doctors also said that from a clinical perspective, “they hope to see the emergence of technology that can assist both the left and right hearts simultaneously”.
In response to this, CoreMedTech has developed an integrated full - magnetic levitation biventricular assist system, DuoCor®, based on the “time - sharing and partitioned axial full - magnetic levitation control technology”. It has now completed more than 10 clinical applications, and its global clinical trials have also been carried out simultaneously.
In addition, in the field of interventional artificial hearts where no products have been approved in China, CoreMedTech's CorVad® interventional artificial heart product, which is developed based on axial flux multi - drive technology, has also achieved a new breakthrough in in - vivo micro - motor technology.
In terms of going global, Corheart 6 has started enrolling patients in clinical trials in Austria and Germany, and the integrated biventricular product DuoCor has also completed its first humanitarian rescue implantation in Hanover, Germany.
CoreMedTech said that the relatively stable market environment and strong medical payment system in Europe will provide an important strategic fulcrum for the company's global layout.
Views of the founder and investors:
Dr. Yu Shunzhou, the founder and CEO of CoreMedTech, said, We are deeply honored to receive the support and recognition of professional investors from all parties. This financing will strongly accelerate the internationalization process of CoreMedTech's full - pipeline products and bring newer and better innovative medical products to heart failure patients around the world.
ZhenFund said, ZhenFund has witnessed the growth of CoreMedTech, which has now become a leading enterprise in the ventricular assist field in China and has the ability to compete in global innovation. ZhenFund highly recognizes the technical height and entrepreneurial spirit of the founding team in global leading innovation in the ventricular assist field, as well as the contributions made by LVAD, BiVAD, and PVAD products to heart failure patients in China and globally, and the company's efficient clinical and commercial execution capabilities.
Legend Capital said: Heart failure is the final battlefield in the cardiovascular field, and the artificial heart is the weapon to meet the urgent and necessary clinical needs. As a senior expert in the global R & D and industrialization of artificial hearts, the company's founder has more than 14 years of industry experience. Starting from clinical needs, the company provides a full - life - cycle solution for heart failure patients, offering more and better choices for the clinical treatment of heart failure patients with a more comprehensive product portfolio.
Prosperity7 Ventures (P7) under Aramco Ventures said, CoreMedTech's product layout and R & D progress in the ventricular assist field fully demonstrate its outstanding strength, which is remarkable. We highly recognize that CoreMedTech starts from clinical needs and builds a full - pipeline product for heart failure circulatory treatment through the high scalability of its technology.
CStone Capital said, As an absolute leader in the Chinese artificial heart industry and a leading enterprise in the international artificial heart field, CoreMedTech has built a rich product pipeline covering the entire field of heart failure treatment through its globally innovative underlying technology breakthroughs in the VAD field. Currently, CoreMedTech's LVAD, BiVAD, and PVAD products have all entered the innovation green channel. As an old shareholder, CStone Capital will continue to accompany the company's growth and firmly recognize the high clinical benefits brought by technological innovation, as well as the high - level innovation spirit and execution ability of Dr. Yu and his team.
DL Capital said, CoreMedTech is an excellent representative enterprise that focuses on underlying technology breakthroughs and develops independent IP products. At the same time, the company has efficient operation management and a clear strategy for going global. The company first created the world's smallest single - left - heart MCS product in a differentiated way, and then expanded to the world's first biventricular and infant MCS products, leading Chinese enterprises to evolve from Best - in - Class to First - in - Class in the global implantable artificial heart field.
LX Capital said, As a leading enterprise in the artificial heart industry, CoreMedTech's first LVAD product achieved the first - place market share breakthrough in the first year of its approval and listing in 2023. The second pVAD product has entered the registration and application stage, and the first LVAD product and the globally innovative integrated full - magnetic levitation BiVAD product under simultaneous R & D have both entered the international clinical stage. LX Capital has continuously participated in each round of the company's financing since the Series A in 2020. We are very honored to cooperate with the company in the long term and jointly promote its continuous innovation and development in the global heart failure treatment field.